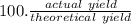Chemistry, 28.11.2020 01:00, augustxnicki
Under a certain set of conditions, the percent yield of a reaction that produces carbon dioxide is 75.0%. What mass in grams of CO2 will actually be recovered if the theoretical yield is 26.7 grams?


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1


Chemistry, 23.06.2019 06:30, fshane7705
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
Do you know the correct answer?
Under a certain set of conditions, the percent yield of a reaction that produces carbon dioxide is 7...
Questions in other subjects:

Computers and Technology, 28.08.2019 19:50




Biology, 28.08.2019 19:50




Health, 28.08.2019 19:50


 %
% 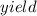 =
=