
Chemistry, 27.11.2020 14:00, lillysiege
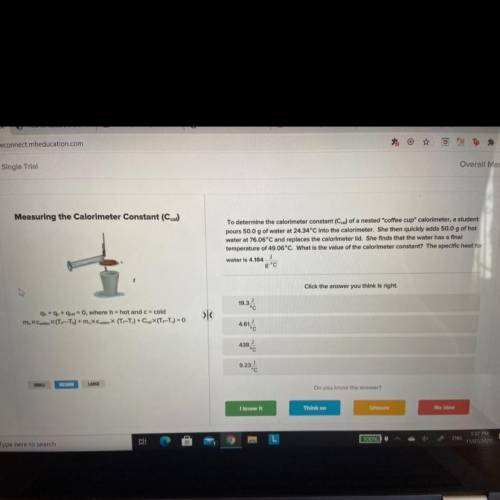
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours 50.0 g of water at 24.34°C into the calorimeter. She then quickly adds 50.0 g of hot
water at 76.06°C and replaces the calorimeter lid. She finds that the water has a final
temperature of 49.06°C. What is the value of the calorimeter constant? The specific heat for
J
water is 4.184

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1



Chemistry, 23.06.2019 09:00, sammypaige08
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
Do you know the correct answer?
To determine the calorimeter constant (Ccal) of a nested "coffee cup" calorimeter, a student
pours...
Questions in other subjects:




History, 21.09.2019 03:30

Mathematics, 21.09.2019 03:30

Chemistry, 21.09.2019 03:30



Business, 21.09.2019 03:30






