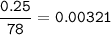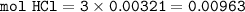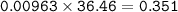Chemistry, 27.11.2020 02:00, carrieaj08
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in antacid tablets neutralizes hydrochloric acid in the stomach. A tablet containing 0.25 g of
aluminum hydroxide is ingested by a patient with 0.88 g of hydrochloric acid in their stomach. Is
this tablet sufficient to neutralize the acid in the patient's stomach? Explain using stoichiometric
calculations. [4 marks]
I

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 23.06.2019 04:31, laurenbreellamerritt
How big are the bighest ocean waves at mavericks
Answers: 1
Do you know the correct answer?
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in ant...
Questions in other subjects:


Mathematics, 03.11.2019 00:31


Mathematics, 03.11.2019 00:31

English, 03.11.2019 00:31

Mathematics, 03.11.2019 00:31

English, 03.11.2019 00:31



Biology, 03.11.2019 00:31