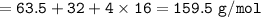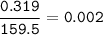Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 23.06.2019 11:30, kayabwaller4589
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1

Do you know the correct answer?
A solution of copper sulfate (CuSO4) produced by bioleaching has a concentration of 0.319 g/dm3 Rela...
Questions in other subjects:

Mathematics, 14.11.2019 07:31






Computers and Technology, 14.11.2019 07:31



Social Studies, 14.11.2019 07:31