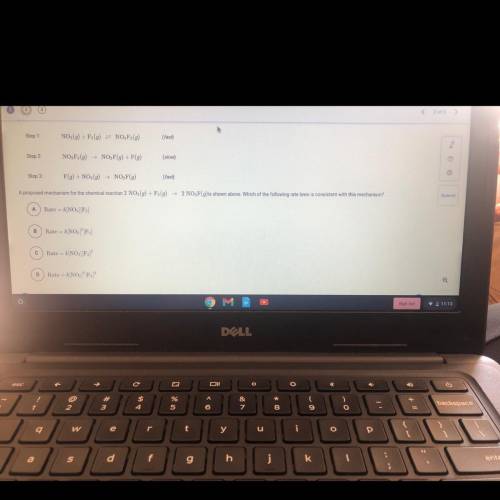
Step 1:
NO2(g) + F2(g) = NO, F2(g)
(fast)
Step 2
NO, F2(9)
NO, F(9) + F(g)<...

Chemistry, 26.11.2020 22:30, ineedhelp2285
Step 1:
NO2(g) + F2(g) = NO, F2(g)
(fast)
Step 2
NO, F2(9)
NO, F(9) + F(g)
(slow)
Step 3:
F(g) + NO (9) NO, F(g)
(fast)
A proposed mechanism for the chemical reaction 2 NO2(g) + F (9)
2 NO, F(g)is shown above. Which of the following rate laws is consistent with this mechanism?
A
Rate = k/NO2][F]
B
Rate = k[NO][F)
С
Rate -- k[NO] F2)
D
Rate
k[NO, F,

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Biology, 15.11.2020 14:00

Physics, 15.11.2020 14:00


Mathematics, 15.11.2020 14:00


Biology, 15.11.2020 14:00

Biology, 15.11.2020 14:00








