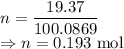I have 2 samples of solid chalk (aka calcium carbonate). Sample A has a total mass of 4.12 g and Sample B has a total mass of 19.37 g. What is the difference between the samples?
A) Sample B has more calcium carbonate molecules
B) Sample B has a larger ratio of carbon, oxygen, and calcium atoms
C) Sample B has more calcium ion than carbonate ions
D) Sample B must have some impurity

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
Do you know the correct answer?
I have 2 samples of solid chalk (aka calcium carbonate). Sample A has a total mass of 4.12 g and Sam...
Questions in other subjects:

Mathematics, 08.01.2021 22:30

Arts, 08.01.2021 22:30

Mathematics, 08.01.2021 22:30

Arts, 08.01.2021 22:30

Mathematics, 08.01.2021 22:30

Mathematics, 08.01.2021 22:30

Mathematics, 08.01.2021 22:30

Social Studies, 08.01.2021 22:30

English, 08.01.2021 22:30

Mathematics, 08.01.2021 22:30


 = Avogadro's number =
= Avogadro's number =