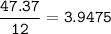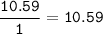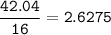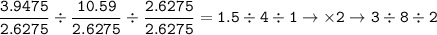Determine the empirical formula of a compound containing 47 37 grams of carbongrams of hydrogen, and of oxygen In an expenmen the me compound was determined to de 228 276 gis the molecular of the compound? For both questions your work or explain how you detemined the formulas by giving specille values used in calculations

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
Do you know the correct answer?
Determine the empirical formula of a compound containing 47 37 grams of carbongrams of hydrogen, and...
Questions in other subjects:


English, 24.03.2021 04:00


Mathematics, 24.03.2021 04:00

History, 24.03.2021 04:00




Biology, 24.03.2021 04:00