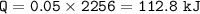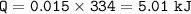PLEASE HELP WITH THESE TWO QUESTIONS
2.) 50.0 grams of water at 100 degrees Celsius evaporated to water vapor 2 points
at 100 degrees Celsius. Calculate the amount of heat required for this
conversion.
2. A student measured 15.0 grams of ice in a beaker. The beaker was then
placed on a hot plate where it was heated uniformly for a certain amount
of time. During the melting process of the ice, the student noted that the
temperature was at 0 degree Celsius. When all the ice converted to water,
the final temperature was also at 0 degree Celsius. How much heat was
used to melt the ice?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, kelyanthecrafte
Which of the following best defines homeostasis? forming identical cells breaking down glucose maintaining stable internal conditions increasing an organism's temperature
Answers: 3

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2
Do you know the correct answer?
PLEASE HELP WITH THESE TWO QUESTIONS
2.) 50.0 grams of water at 100 degrees Celsius evaporated to w...
Questions in other subjects:

History, 30.09.2019 23:30

Computers and Technology, 30.09.2019 23:30

History, 30.09.2019 23:30

Mathematics, 30.09.2019 23:30

English, 30.09.2019 23:30


Social Studies, 30.09.2019 23:30

Biology, 30.09.2019 23:30


Mathematics, 30.09.2019 23:30