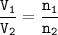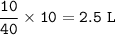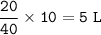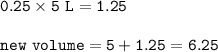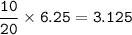The chemical equilibrium is given:
2NO (g) + O2(g) <—>2N O2, (g)
In a container...

Chemistry, 25.11.2020 14:00, shartman22
The chemical equilibrium is given:
2NO (g) + O2(g) <—>2N O2, (g)
In a container of volume 10L is in equilibrium a mixture consisting of 10 mol NO (g), 10 mol O2, (g) and 20 mol NO2, (g).
The volume of the container changes under constant temperature and after the restoration of equilibrium the amount of NO has increased by 25%. Calculate the volume change in L.
How do I find the change in volume?
P. s sorry if my English isn’t perfect.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Do you know the correct answer?
Questions in other subjects:

Spanish, 29.01.2021 19:50

Mathematics, 29.01.2021 19:50


English, 29.01.2021 19:50

Mathematics, 29.01.2021 19:50




Mathematics, 29.01.2021 19:50