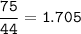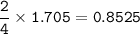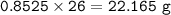Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 10:30, piratesfc02
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
Do you know the correct answer?
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces
carbon dio...
Questions in other subjects:

Mathematics, 23.01.2021 01:00

English, 23.01.2021 01:00




Social Studies, 23.01.2021 01:00

Mathematics, 23.01.2021 01:00


Mathematics, 23.01.2021 01:00

English, 23.01.2021 01:00