Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, Staceyrycz2772
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
Do you know the correct answer?
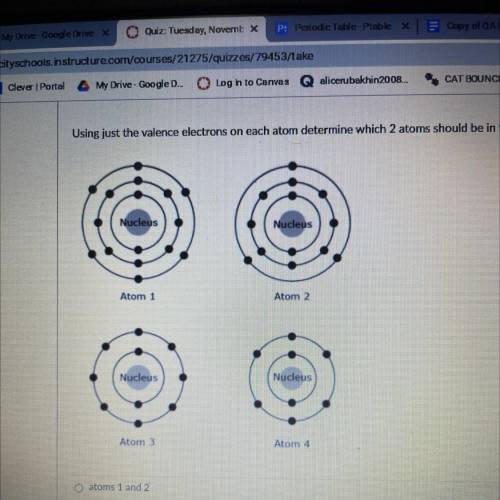
Using just the valence electrons on each atom determine which 2 atoms should be in the same group?...
Questions in other subjects:

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Social Studies, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

Mathematics, 14.09.2020 16:01

History, 14.09.2020 16:01

Computers and Technology, 14.09.2020 16:01

English, 14.09.2020 16:01