
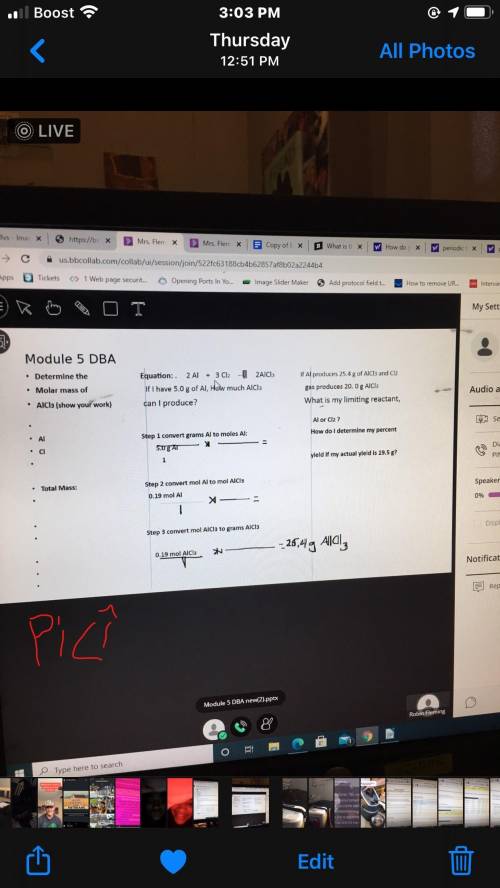
2Al+3Cl2---> 2AlCl3 (i cant remember if this is balanced equation or not if not can you do that too? thank you i appreciate it.)
If I have 5.0 g Aluminum how much AlCl can I produce?
Step 1 convert grams Al to moles Al: (the dashes resemble a fraction)
5.0 g Al
- times - =
Step 2: convert mol al to mol AlCl3
0.19 mol Al
- times - =
1
step 3: convert MOL AlCl3 to GRAMS AlCl3
0.19 mol AlCl3
- times - = 25.4 g AlCl3
1
THIS FIRST # PART PROBLEM IS STOICHEMITRY for chemistry if you know please help me out I have been struggling on this for a week now..thank you so much.
This next part is LIMITING REACTANTS:
If Al produces 25.4 g of AlCl3 and Cl2 gas produces 20.0 g AlCl3 what is my limiting reactant, Al or C2??
How do i determine my percent yeild if my actual yeild is 19.5g???
i put a link to the picture!!

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
Do you know the correct answer?
2Al+3Cl2---> 2AlCl3 (i cant remember if this is balanced equation or not if not can you do that t...
Questions in other subjects:


English, 27.10.2020 23:30


Engineering, 27.10.2020 23:30


Mathematics, 27.10.2020 23:30

History, 27.10.2020 23:30

Social Studies, 27.10.2020 23:30


Social Studies, 27.10.2020 23:30






