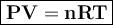Chemistry, 24.11.2020 05:40, CameronVand21
0.075 grams of condensed vapor is present
when a student uses the Dumas method. The
experiment was conducted at 70°C, 1 atm and
the volume of the container is 0.045 L. What is
the molar mass of the material?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2


Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
Do you know the correct answer?
0.075 grams of condensed vapor is present
when a student uses the Dumas method. The
experimen...
experimen...
Questions in other subjects:

Mathematics, 30.10.2020 16:30





History, 30.10.2020 16:30





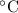 = 343.15 K
= 343.15 K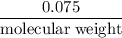 × 0.08206 × 343.15
× 0.08206 × 343.15