

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
Do you know the correct answer?
. Laughing gas is used by dentists as an anesthetic. If a 10.0 L tank of laughing gas contains 1.26...
Questions in other subjects:


Mathematics, 09.01.2020 22:31


English, 09.01.2020 22:31



Arts, 09.01.2020 22:31


Mathematics, 09.01.2020 22:31

History, 09.01.2020 22:31


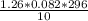 = 3.058atm
= 3.058atm



