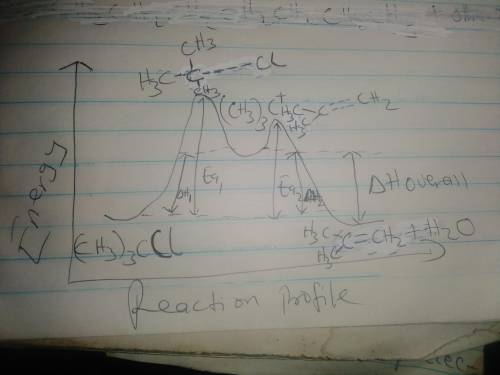
The conversion of (CH3)3CI to (CH3)2C=CH2 can occur
by either one-step or two-step mechanism, as shown in
Equations (1) and (2]
[1]
있
+ I + H₂O
HK
OH
[2]
07
Slow
+ H₂O
+ I
f) Assume Equation [a] represents an endothermic reaction
and that the product of the rate determining step is
higher in energy than the reactants or products.
Draw an energy dagram for this two-step reaction.
Label the axes, reactants and products for each step,
and the Ea and 4to for each step. Label 44° overall.
Draw the structure for both transition states.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
Do you know the correct answer?
The conversion of (CH3)3CI to (CH3)2C=CH2 can occur
by either one-step or two-step mechanism, as sh...
Questions in other subjects:



English, 03.07.2019 22:30


Biology, 03.07.2019 22:30


English, 03.07.2019 22:30


Chemistry, 03.07.2019 22:30