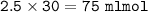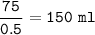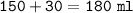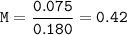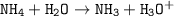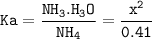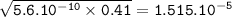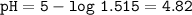Chemistry, 23.11.2020 08:50, raynamg2718
Just enough 0.500 M HCl is added to 30.0 mL of 2.5 M NH3 to reach the equivalence point. The Kb of NH3 = 1.8 X 10-5
Write the balanced equation for this reaction.
What volume of 0.500 M HCl solution was added?
What is the molarity of the salt produced from the neutralization reaction?
What is the pH of the solution at the equivalence point?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 23.06.2019 02:50, giavanleer14
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3
Do you know the correct answer?
Just enough 0.500 M HCl is added to 30.0 mL of 2.5 M NH3 to reach the equivalence point. The Kb of N...
Questions in other subjects:

English, 17.04.2021 06:10


Mathematics, 17.04.2021 06:10






English, 17.04.2021 06:10