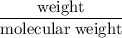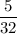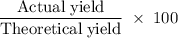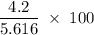Chemistry, 23.11.2020 04:10, corcoranrobert1959
A sample of 5.0 g of hydrogen gas reacts with 5.0 g of oxygen gas.
2H2(g) + O2(g) > 2H2O
Please answer the questions in a b c d and e, or at least in any of the ones you know. Thank you.


Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
Do you know the correct answer?
A sample of 5.0 g of hydrogen gas reacts with 5.0 g of oxygen gas.
2H2(g) + O2(g) > 2H2O
Questions in other subjects:


History, 05.02.2021 02:10



English, 05.02.2021 02:10


Mathematics, 05.02.2021 02:10



History, 05.02.2021 02:10



 2
2