
Chemistry, 22.11.2020 09:00, ryanbransky
Silver iodide powder has been used as an antiseptic and as an agent to seed clouds for rain. Silver iodide is 45.9% silver by mass. If you separate a 50.0-g sample of silver iodide into its elements, silver and iodine, how much silver would you have?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 23.06.2019 07:00, bree6754
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
Do you know the correct answer?
Silver iodide powder has been used as an antiseptic and as an agent to seed clouds for rain. Silver...
Questions in other subjects:

Health, 18.01.2020 02:31


Social Studies, 18.01.2020 02:31



Biology, 18.01.2020 02:31

Geography, 18.01.2020 02:31

French, 18.01.2020 02:31

English, 18.01.2020 02:31


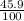 x 50 = 22.95g
x 50 = 22.95g



