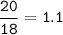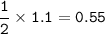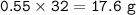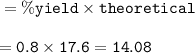Chemistry, 21.11.2020 21:00, Scotdouglas346
20.0 grams of water decomposes into hydrogen gas and oxygen gas through electrolysis. If the percent yield is 80.0%, what mass of oxygen gas is produced?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:50, kelli151
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2


Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
Do you know the correct answer?
20.0 grams of water decomposes into hydrogen gas and oxygen gas through electrolysis. If the percent...
Questions in other subjects:

Mathematics, 16.04.2021 03:40

History, 16.04.2021 03:40

Biology, 16.04.2021 03:40



Computers and Technology, 16.04.2021 03:40



Mathematics, 16.04.2021 03:40

Mathematics, 16.04.2021 03:40