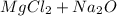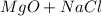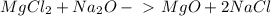For questions #14 – 17, write an equation for the reaction of magnesium chloride and sodium oxide to produce magnesium oxide with sodium chloride.
14. show the formulas of the reactants.
15. show the formulas of the products.
16. write the balanced the equation for this reaction.
17. what type of chemical reaction is this? how do you know?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1


Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
Do you know the correct answer?
For questions #14 – 17, write an equation for the reaction of magnesium chloride and sodium oxide to...
Questions in other subjects:

History, 24.09.2020 16:01


Mathematics, 24.09.2020 16:01

English, 24.09.2020 16:01

Mathematics, 24.09.2020 16:01

Mathematics, 24.09.2020 16:01

History, 24.09.2020 16:01