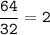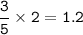Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
Do you know the correct answer?
Given the unbalanced combustion equation: C3H8 + O2 → CO2 + H2O, how many moles of carbon dioxide ar...
Questions in other subjects:








Chemistry, 02.12.2020 18:40

Geography, 02.12.2020 18:40

English, 02.12.2020 18:40