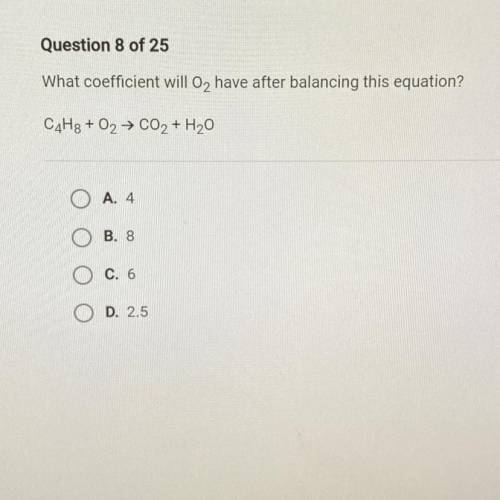
What coefficient well O2 have after balancing this equation
C4H8 + O2 -> CO2 + H2O
A...


Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
Do you know the correct answer?
Questions in other subjects:



English, 25.01.2020 14:31

Mathematics, 25.01.2020 14:31

Social Studies, 25.01.2020 14:31

Mathematics, 25.01.2020 14:31


Mathematics, 25.01.2020 14:31

Mathematics, 25.01.2020 14:31

History, 25.01.2020 14:31