
Chemistry, 20.11.2020 21:50, estefaniapenalo
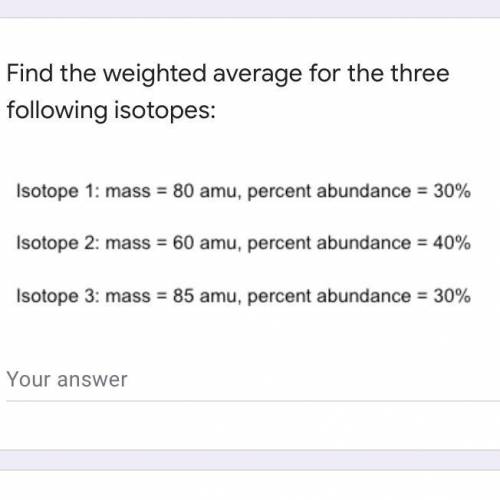
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abundance dance=30\% Isotope 2: mass = 60 amu, percent 1dance=40\% Isotope 3: mass = 85 amu, percent abundance 1dance=30\%

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
Do you know the correct answer?
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abunda...
Questions in other subjects:


History, 01.06.2021 07:40


History, 01.06.2021 07:40

Chemistry, 01.06.2021 07:40


World Languages, 01.06.2021 07:50








