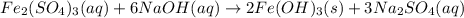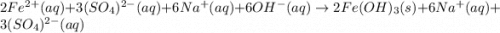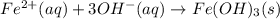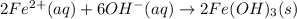Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, momof7hardings
When would a bouncy ball have the most potential energy
Answers: 2

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
An aqueous solution of Iron(III) sulfate, Fe2(SO4)3, is mixed with an aqueous solution of Sodium hyd...
Questions in other subjects:

Mathematics, 17.05.2021 18:50


Mathematics, 17.05.2021 18:50

Biology, 17.05.2021 18:50

History, 17.05.2021 18:50