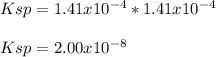Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, haileywebb8
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
Do you know the correct answer?
The pH of a saturated solution of a metal hydroxide MOH is 10.15. Calculate the Ksp for this compoun...
Questions in other subjects:




Mathematics, 11.10.2020 06:01

English, 11.10.2020 06:01


Biology, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01

Biology, 11.10.2020 06:01


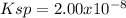
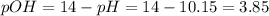
![[OH^-]=10^{-pOH}=10^{-3.95}=1.41x10^{-4}M](/tpl/images/0917/3576/8b4bb.png)
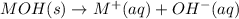
![Ksp=[M^+][OH^-]](/tpl/images/0917/3576/0c3be.png)