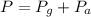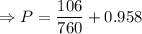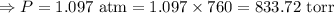Chemistry, 20.11.2020 14:00, rairaibabycakez9578
The mercury level in the open arm of an open-end manometer is 106 mm above the level in the arm connected to a tank of gas. If the barometric pressure is 0.958 atm, what is the pressure (in torr) of the gas in the tank?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, hannahkelly3618
How many moles are equivalent to 55.5g of nano3
Answers: 1


Chemistry, 23.06.2019 02:50, agm0102
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3

Chemistry, 23.06.2019 08:40, sugakookies1
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
Do you know the correct answer?
The mercury level in the open arm of an open-end manometer is 106 mm above the level in the arm conn...
Questions in other subjects:



Mathematics, 07.03.2020 05:03


Mathematics, 07.03.2020 05:03






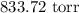
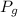 = Pressure of gas =
= Pressure of gas = 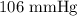
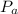 = Atmospheric pressure = 0.958 atm
= Atmospheric pressure = 0.958 atm