
Chemistry, 20.11.2020 14:00, eboniwiley
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. grams of water at 25.0 celsius. when the system comes to equilibrium the temperature of the system is 35.0 celsius. what is the mass in g of the iron? assume no heat is lost to the surroundingsSpecific heat/J. g^-1 K^-1iron 0.450water 4.184 (A) 4.48 g (B) 41.7 g (C) 387 g (D) 612 g

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 06:30, aurikmah2005
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
Do you know the correct answer?
A piece of iron is heated to 95.0 celsius and then placed in an insulated vessel containing 250. gra...
Questions in other subjects:

Arts, 03.11.2020 22:40

Physics, 03.11.2020 22:40

Spanish, 03.11.2020 22:40

Health, 03.11.2020 22:40

History, 03.11.2020 22:40

Biology, 03.11.2020 22:40



Arts, 03.11.2020 22:40

Mathematics, 03.11.2020 22:40


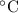 × (308 K - 298 K)
× (308 K - 298 K)



