
Chemistry, 20.11.2020 07:20, tdluong157
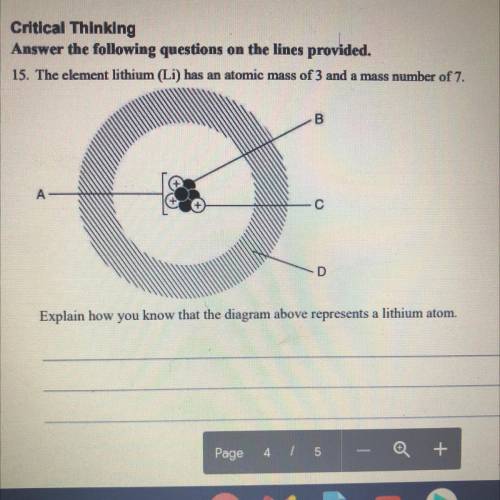
15. The element lithium (Li) has an atomic mass of 3 and a mass number of 7. Explain how you know that the diagram above represents a lithium atom.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2


Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1

Chemistry, 23.06.2019 11:00, jdisalle2808
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
Do you know the correct answer?
15. The element lithium (Li) has an atomic mass of 3 and a mass number of 7.
Explain how you know...
Questions in other subjects:



Mathematics, 22.07.2019 00:32


English, 22.07.2019 00:32


Spanish, 22.07.2019 00:32








