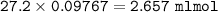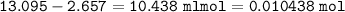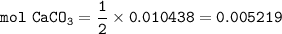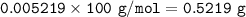Chemistry, 18.11.2020 21:30, lilpeepxliltracy
1.3188g of antacid is weighed and mixed with 75.00 mL of excess 0.1746 M HCl. The excess wcid required 27.20 mL of 0.09767 M NaOH for back titration. Calculate the amount of CaCO3 in the tablet.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2


Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1
Do you know the correct answer?
1.3188g of antacid is weighed and mixed with 75.00 mL of excess 0.1746 M HCl. The excess wcid requir...
Questions in other subjects:

English, 02.07.2019 01:40

Chemistry, 02.07.2019 01:40


History, 02.07.2019 01:40


History, 02.07.2019 01:40



Mathematics, 02.07.2019 01:40