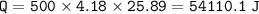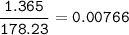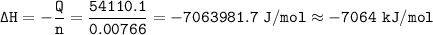When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket containing 500.0 g of water, the temperature of the water increases by 25.89°C. Assuming that the specific heat of water is 4.18 J/(g ∙°C), and that the heat absorption by the calorimeter is negligible, estimate the enthalpy of combustion per mole of anthracene.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2


Chemistry, 23.06.2019 04:31, saladdressing1425
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
Do you know the correct answer?
When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket conta...
Questions in other subjects:



Geography, 22.01.2021 19:50



Mathematics, 22.01.2021 19:50

Physics, 22.01.2021 19:50