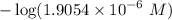Chemistry, 18.11.2020 17:10, erinwebsterrr
50.0 mL of 0.200 M HNO2 is titrated to its equivalence point with 1.00 M NaOH. What is the pH at the equivalence point?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, willcohen42
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3
Do you know the correct answer?
50.0 mL of 0.200 M HNO2 is titrated to its equivalence point with 1.00 M NaOH. What is the pH at the...
Questions in other subjects:


English, 22.09.2021 14:00

Biology, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

Physics, 22.09.2021 14:00

History, 22.09.2021 14:00


Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00




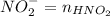
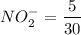

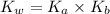
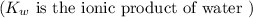
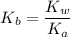
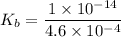
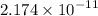
![$K_b = \frac{\left[HNO_2\right] \left[OH^- \right]}{\left[NO^-_2 \right]}$](/tpl/images/0908/6965/f3dc7.png)

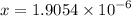
![$[OH^-]=1.9054 \times 10^{-6 } \ M$](/tpl/images/0908/6965/e9392.png)
![$\log[OH^-]$](/tpl/images/0908/6965/5a237.png)