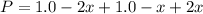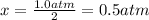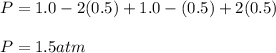Chemistry, 18.11.2020 17:10, juliah6925
Consider the generic reaction: 2 A(g) + B(g) → 2 C(g). If a flask initially contains 1.0 atm of A and 1.0 atm of B, what is the pressure in the flask if the reaction proceeds to completion? (Assume constant volume and temperature.)a. 1.0 atmb. 1.5 atmc. 2.0 atmd. 3.0 atm

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, raymond5799
What is the h+ concentration for an aqueous solution with poh = 4.01 at 25 ∘c? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3


Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
Do you know the correct answer?
Consider the generic reaction: 2 A(g) + B(g) → 2 C(g). If a flask initially contains 1.0 atm of A an...
Questions in other subjects:

Chemistry, 07.07.2021 23:00

Mathematics, 07.07.2021 23:00


Mathematics, 07.07.2021 23:00


Mathematics, 07.07.2021 23:00

Computers and Technology, 07.07.2021 23:00



Mathematics, 07.07.2021 23:00


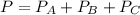
 we can write:
we can write: