
Chemistry, 18.11.2020 16:50, Conner4600
PLEASEEE HELPPP
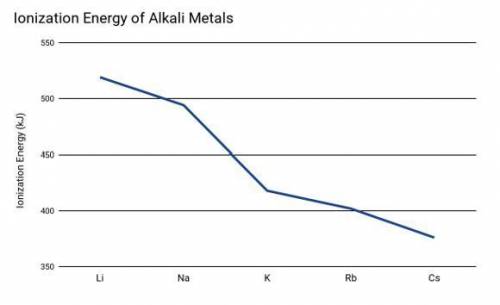
Two students were conducting and experiment based on the ionization energy of alkali metals. The driving question for the experiment was: "What is the relationship between ionization energy and the rate of reaction (time for the reaction to be completed) using alkali metals."
Which of the following predictions would be the best option based on the student's prior knowledge learned and information provided in the graph above?
A.
Cesium will have a higher rate of reaction (faster) because it has the lowest ionization energy which indicates a higher reactivity.
B.
Cesium will have a lower rate of reaction (slower) because it has the lowest ionization energy which indicates a lower reactivity.
C.
The ionization energy of the alkali metals will not affect the rate of the reaction because the energy required to remove an electron does not affect time.
D.
Lithium will have a higher rate of reaction (faster) because it has the highest ionization energy which indicates a higher reactivity.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 05:30, MansellS5529
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3
Do you know the correct answer?
PLEASEEE HELPPP
Two students were conducting and experiment based on the ionization energy of alkal...
Questions in other subjects:




Social Studies, 09.12.2021 04:20











