
Copper metal (Cu) reacts with silver nitrate (AgNO3) in aqueous solution to form Ag and Cu(NO3)2. An excess of AgNO3 is present. The balanced chemical equation is shown below.
Cu + 2AgNO3 Right arrow. Cu(NO3)2 + 2Ag
The molar mass of Cu is 63.5 g/mol. The molar mass of Ag is 107.9 g/mol. What mass, in grams, of Ag is produced from reaction of 31.75 g of Cu?
26.95
107.9
215.91
431.82

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 23.06.2019 00:30, coralaguilar1702
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 01:00, dawnparker71
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 03:00, mullerma
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
Do you know the correct answer?
Copper metal (Cu) reacts with silver nitrate (AgNO3) in aqueous solution to form Ag and Cu(NO3)2. An...
Questions in other subjects:




History, 26.09.2019 06:30

English, 26.09.2019 06:30

Chemistry, 26.09.2019 06:30


Mathematics, 26.09.2019 06:30

Mathematics, 26.09.2019 06:30

Mathematics, 26.09.2019 06:30


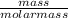
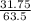 = 0.5moles
= 0.5moles 



