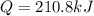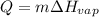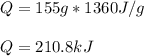Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2

Do you know the correct answer?
The Heat of vaporization for NH3= 1360 J/g. Calculate the quantity of heat energy (in kJ)
needed to...
Questions in other subjects:

French, 24.03.2021 14:00


Mathematics, 24.03.2021 14:00


English, 24.03.2021 14:00


Mathematics, 24.03.2021 14:00


Mathematics, 24.03.2021 14:00

English, 24.03.2021 14:00