
Chemistry, 17.11.2020 17:30, rodriguezbrian050702
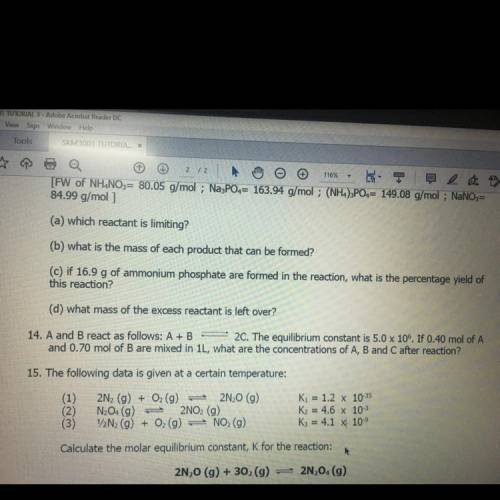
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A and 0.70 mol of B are mixed in 1L, what are the concentrations of A, B and C after reaction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 21.06.2019 22:30, bartfrank447
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2
Do you know the correct answer?
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A
and 0...
Questions in other subjects:





Mathematics, 24.01.2022 04:00

Mathematics, 24.01.2022 04:00




Mathematics, 24.01.2022 04:00






