
Chemistry, 17.11.2020 09:40, mdndndndj7365
If the pressure exerted by a gas at 25 degrees C in a volume of 0.044 L is 3.81 atm, how many moles of gas are present?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, monsurviky
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 21.06.2019 22:20, Brooke7644
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Do you know the correct answer?
If the pressure exerted by a gas at 25 degrees C in a volume of 0.044 L is 3.81 atm, how
many moles...
Questions in other subjects:

Mathematics, 06.11.2020 23:10




History, 06.11.2020 23:10

Mathematics, 06.11.2020 23:10



Geography, 06.11.2020 23:10

Arts, 06.11.2020 23:10


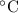 = 298 K
= 298 K 0.044 = n
0.044 = n 



