
Chemistry, 17.11.2020 01:00, JvGaming2001
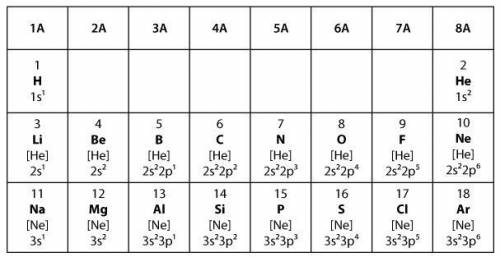
Covalent bonds form when two elements share electrons to make a complete outer shell of electrons. Ionic bonds form when one element donates one or more electrons to another element to make a complete outer shell of electrons.
Which statement best explains the type of bond that will form between two elements from group 6A in the model?
A
The two elements will form a covalent bond because both elements will share a single electron in order to have full outer shells.
B
The two elements will form a covalent bond because both elements will share a pair of electrons in order to have full outer shells.
C
The two elements will form an ionic bond because one of the elements will donate one electron to the other element in order to have full outer shells.
D
The two elements will form an ionic bond because one of the elements will donate two electrons to the other element in order to have full outer shells.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, officialgraciela67
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1


Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
Do you know the correct answer?
Covalent bonds form when two elements share electrons to make a complete outer shell of electrons. I...
Questions in other subjects:

Mathematics, 19.07.2019 20:00



Physics, 19.07.2019 20:00

Social Studies, 19.07.2019 20:00











