

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, familyvazquez7
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 11:40, Wemaybewrong
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
Do you know the correct answer?
One of the light waves produced when hydrogen is energized has a wavelength of 4.105 x10^-7 m. What...
Questions in other subjects:



Physics, 30.12.2021 14:00



SAT, 30.12.2021 14:00


SAT, 30.12.2021 14:00



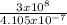 = 7.3 x 10¹⁴Hz
= 7.3 x 10¹⁴Hz



