
Chemistry, 14.11.2020 09:40, franvelpaulino2191
A 2.70g vitamin C tablet is found to contain 0.0109 mol of
ascorbic acid (C6H806}. The molar mass of C6H806 is 176.12 g/mol. What
is the mass percent of C6H806 in the tablet?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2
Do you know the correct answer?
A 2.70g vitamin C tablet is found to contain 0.0109 mol of
ascorbic acid (C6H806}. The molar mass o...
Questions in other subjects:

History, 17.09.2019 00:30


Mathematics, 17.09.2019 00:30

English, 17.09.2019 00:30

History, 17.09.2019 00:30

Mathematics, 17.09.2019 00:30


Mathematics, 17.09.2019 00:30

Health, 17.09.2019 00:30


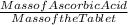 ) * 100
) * 100



