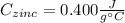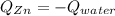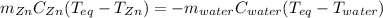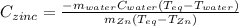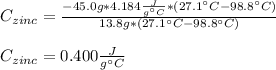Chemistry, 13.11.2020 20:00, missjohnson4449
A 13.8 g of zinc is heated to 98.8 c in boiling water and then dropped onto a beaker containing 45.0 g of water at 25.o °C .when the water and metal come to thermal equilibrium the temperature is 27.1°C .what is the specific heat capacity

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, sairaanwar67
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2


Do you know the correct answer?
A 13.8 g of zinc is heated to 98.8 c in boiling water and then dropped onto a beaker containing 45.0...
Questions in other subjects:


History, 23.09.2019 08:00



Mathematics, 23.09.2019 08:00


Geography, 23.09.2019 08:00


English, 23.09.2019 08:00