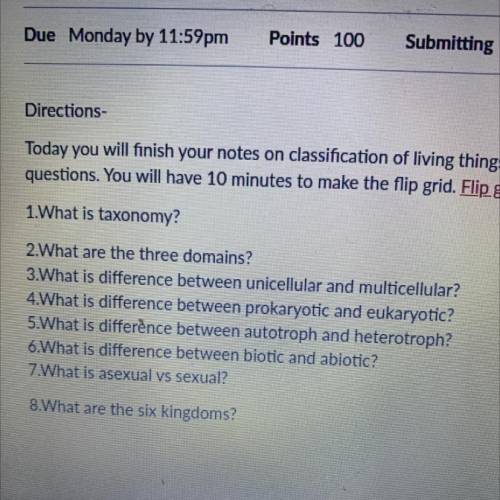
I need help pretty bad can you answer all these questions
...

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, jivsf
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Social Studies, 13.08.2020 21:01

Mathematics, 13.08.2020 21:01

Mathematics, 13.08.2020 21:01



Mathematics, 13.08.2020 21:01



English, 13.08.2020 21:01