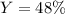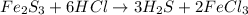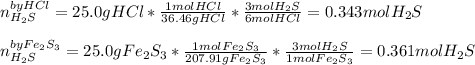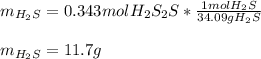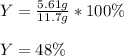Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Do you know the correct answer?
If 25.0g solid iron (III) sulfide reacts with 25.0g gaseous hydrogen chloride to form solid iron (II...
Questions in other subjects:


English, 05.05.2020 16:49

English, 05.05.2020 16:49

Biology, 05.05.2020 16:49

History, 05.05.2020 16:49