
Chemistry, 12.11.2020 20:30, michael1295
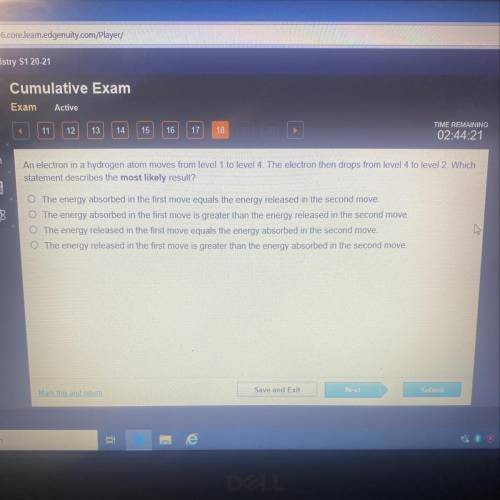
An electron in a hydrogen atom moves from level 1 to level 4. The electron then drops from level 4 to level 2. Which
statement describes the most likely result?
The energy absorbed in the first move equals the energy released in the second move.
O The energy absorbed in the first move is greater than the energy released in the second move,
The energy released in the first move equals the energy absorbed in the second move.
The energy released in the first move is greater than the energy absorbed in the second move.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, khalaflaf2684
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 23.06.2019 01:00, kwarwick0915
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
Do you know the correct answer?
An electron in a hydrogen atom moves from level 1 to level 4. The electron then drops from level 4 t...
Questions in other subjects:


Biology, 17.10.2019 06:30




Spanish, 17.10.2019 06:30



English, 17.10.2019 06:30

Health, 17.10.2019 06:30






