Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, suzymott1562
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1
Do you know the correct answer?
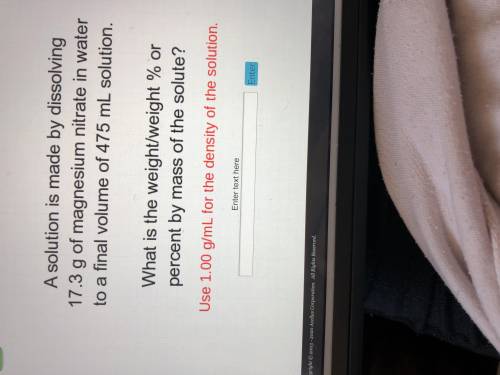
A solution is made by dissolving 17.3 g of magnesium nitrate in water to a final volume of 475 mL so...
Questions in other subjects:


History, 06.10.2019 08:30




History, 06.10.2019 08:30



Mathematics, 06.10.2019 08:30