
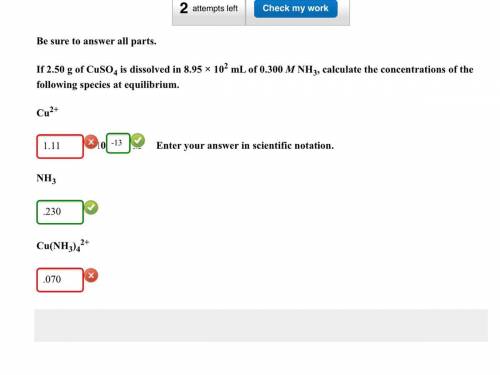
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the following species at equilibrium: Cu^2+, NH3, Cu(NH3)4^2.
I tried to solve and came up with the following-
Cu^2+ = 1.1149x10^-13 (Which was all wrong except for the 10^-13)
NH3 = .230 (Which was correct)
Cu(NH3)4^2+ = 0.069720 (Which was wrong)
Can someone please show me where I am going wrong.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
Do you know the correct answer?
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the...
Questions in other subjects:


Mathematics, 29.04.2021 21:20


Mathematics, 29.04.2021 21:20


English, 29.04.2021 21:20



Mathematics, 29.04.2021 21:20






