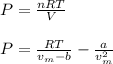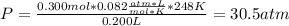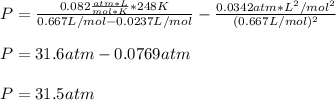Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 16:30, kitykay2776
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
Do you know the correct answer?
For a 0.300 mol sample of helium gas in a 0.200 L container at 248K, will the pressure be greater if...
Questions in other subjects: