
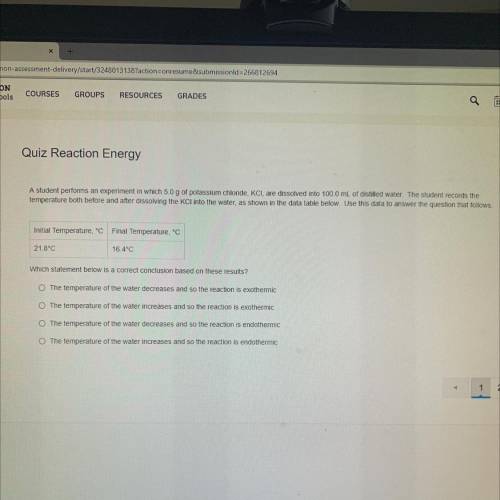
A student performs an experiment in which 5.0 g of potassium chloride, KCI, are dissolved into 100.0 mL of distilled water. The student records the
temperature both before and after dissolving the KCl into the water, as shown in the data table below. Use this data to answer the question that follows.
Initial Temperature, °C Final Temperature, °C
21.8°C
16.4°C
Which statement below is a correct conclusion based on these results ?
The temperature of the water decreases and so the reaction is exothermic
The temperature of the water increases and so the reaction is exothermic
The temperature of the water decreases and so the reaction is endothermic
The temperature of the water increases and so the reaction is endothermic

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2



Chemistry, 23.06.2019 21:30, officialrogerfp3gf2s
If 1.00 mol cs2 reacts with 1.00 mol o2, identify the limiting reactions
Answers: 3
Do you know the correct answer?
A student performs an experiment in which 5.0 g of potassium chloride, KCI, are dissolved into 100.0...
Questions in other subjects:



Chemistry, 11.04.2020 01:50

Mathematics, 11.04.2020 01:50

Mathematics, 11.04.2020 01:50

Mathematics, 11.04.2020 01:50

English, 11.04.2020 01:50

Law, 11.04.2020 01:50








