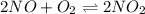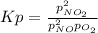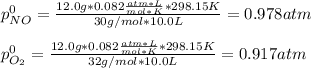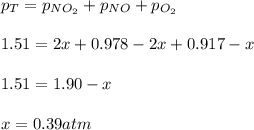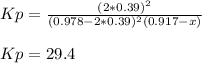Chemistry, 11.11.2020 17:50, dillon3466
For the reaction of nitric oxide and oxygen to form nitrogen dioxide, the reaction begins with 12.0 g of nitric oxide and 12.0 g of oxygen at 25oC in a 10.0 L container. At equilibrium, the pressure in the container is 1148 mmHg, what is Kp?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
Do you know the correct answer?
For the reaction of nitric oxide and oxygen to form nitrogen dioxide, the reaction begins with 12.0...
Questions in other subjects:

Mathematics, 07.11.2020 14:00



History, 07.11.2020 14:00

Mathematics, 07.11.2020 14:00



Social Studies, 07.11.2020 14:00