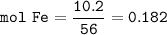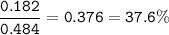Chemistry, 11.11.2020 06:10, connienash95
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a) Actual yield of Fe mole
(b) % mole
(c) theoretical yield of iron mmoles

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Do you know the correct answer?
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a)...
(a)...
Questions in other subjects:


Mathematics, 30.11.2021 19:30





History, 30.11.2021 19:30



Biology, 30.11.2021 19:30